Important Safety Information
For topical dermatologic use only. Not for oral, ophthalmic, or intravaginal use.
OXISTAT® (oxiconazole nitrate) Lotion is contraindicated in individuals who have shown hypersensitivity to any of its components. If a reaction suggesting sensitivity or chemical irritation should occur with the use of OXISTAT Lotion, treatment should be discontinued and appropriate therapy instituted. If signs of epidermal irritation should occur, the drug should be discontinued.In a clinical trial with OXISTAT Lotion (N=269), the most common adverse events were burning and stinging (0.7% each) and pruritus, scaling, tingling, pain and dyshidrotic eczema (0.4% each).
The following additional adverse experiences have been reported with the topical use of oxiconazole nitrate: irritation and allergic contact dermatitis, folliculitis, erythema, papules, fissure, maceration, rash, and nodules.
You are encouraged to report negative side effects of prescription drugs to the FDA. Please visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Indication
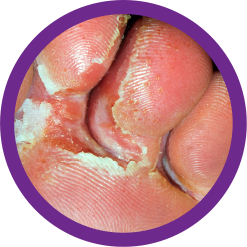
OXISTAT Lotion is indicated for the topical treatment of the following dermal infections: tinea pedis, tinea cruris, and tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, or Epidermophyton floccosum.
Click here to see full Prescribing Information.

The information on this site is intended for U.S. residents only.
OXISTAT® is a registered trademark of Fougera Pharmaceuticals, Inc.
© 2023 ANI Pharmaceuticals, Inc. All rights reserved.
Site Map
| Terms of Use |
Privacy Policy |
ANI Pharmaceuticals, Inc
The information on this site is intended for U.S. residents only.
10470 Rev 03/23