Apply the power of OXISTAT Lotion
OXISTAT Lotion demonstrated greater efficacy in tinea pedis vs vehicle when dosed bid and qd1,2
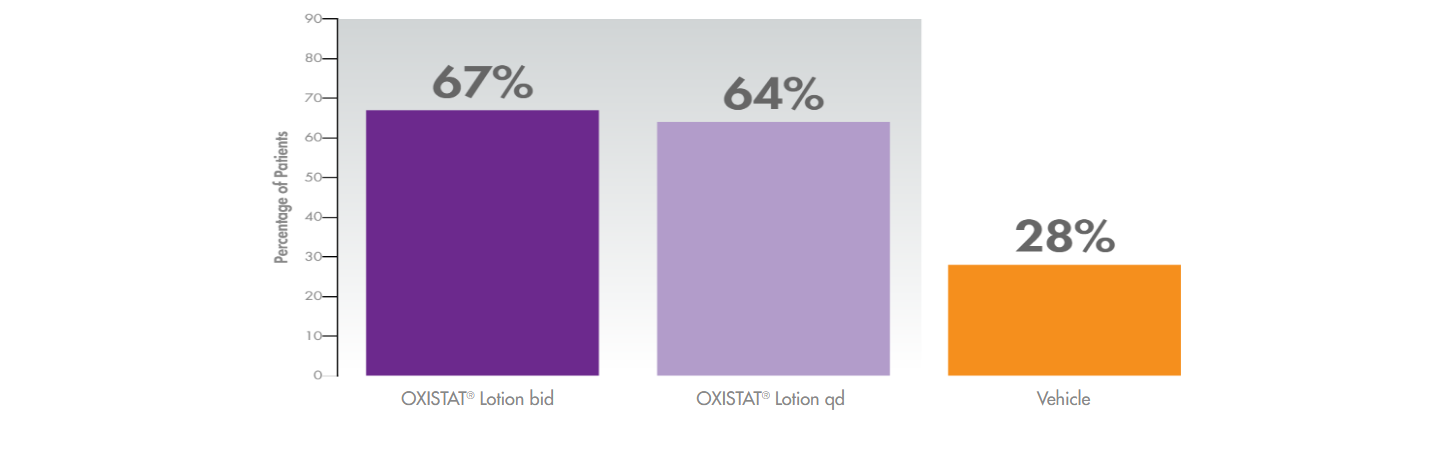
Higher mycological cure rates with OXISTAT Lotion in tinea pedis vs vehicle*1,2
(demonstrated 2 weeks post 4-week treatment)
Mycological cure1
- No evidence (culture and potassium hydroxide [KOH] preparation) of the original pathogen
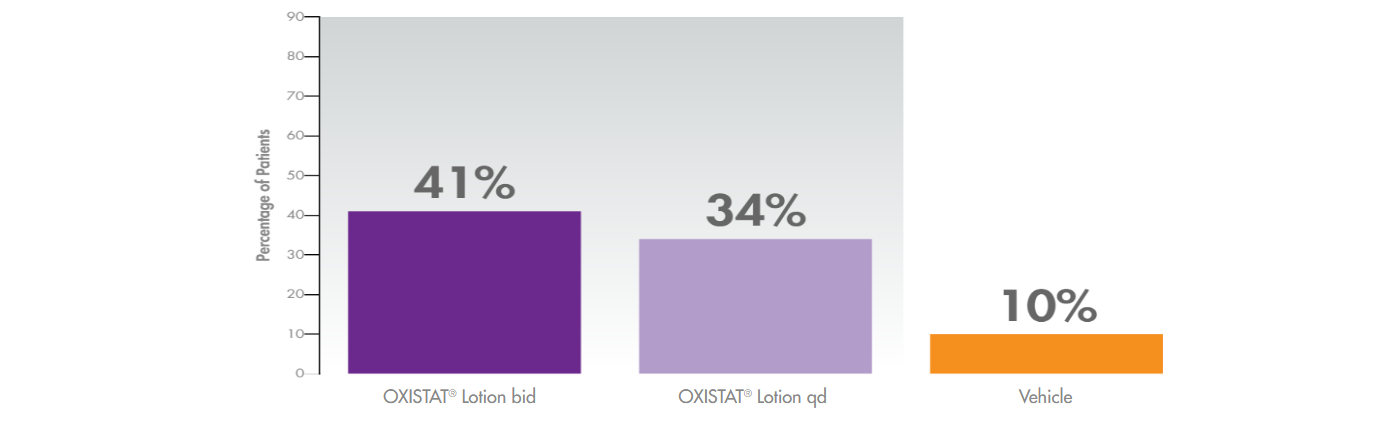
Higher rates of treatment success with OXISTAT Lotion in tinea pedis vs vehicle*1,2
(demonstrated 2 weeks post 4-week treatment)
Treatment success1
- Global evaluation of 90% clinical improvement
- Microbiological eradication
In a clinical trial of 269 patients treated with OXISTAT Lotion, the most common adverse events were burning and stinging (0.7% each) and pruritus, scaling, tingling, pain, and dyshidrotic eczema (0.4% each).1
* In a clinical trial of OXISTAT Lotion involving 332 evaluable patients with clinically and microbiologically established tinea pedis, OXISTAT Lotion showed no trace of fungal infections in lab tests (in 67% when used twice daily and 64% when used once daily, compared with 28% patients using lotion without medicine) and almost complete symptom improvement and no trace of fungal infection in lab tests (in 41% when used twice daily and 34% when used once daily, compared with 10% of patients using lotion without medicine).1
References: 1. OXISTAT Lotion Prescribing Information; July 2022. 2. Data on file, ANI Pharmaceuticals, Inc.